Zydus Lifesciences pulled up by FDA over quality-control issues
The US health regulator, in a letter to the pharma company, accuses it of violating good manufacturing practices at its Vadodara plant.
15 September, 2024•2 min
0
15 September, 2024•2 min
0
Getting your Trinity Audio player ready...

More in Business
Business
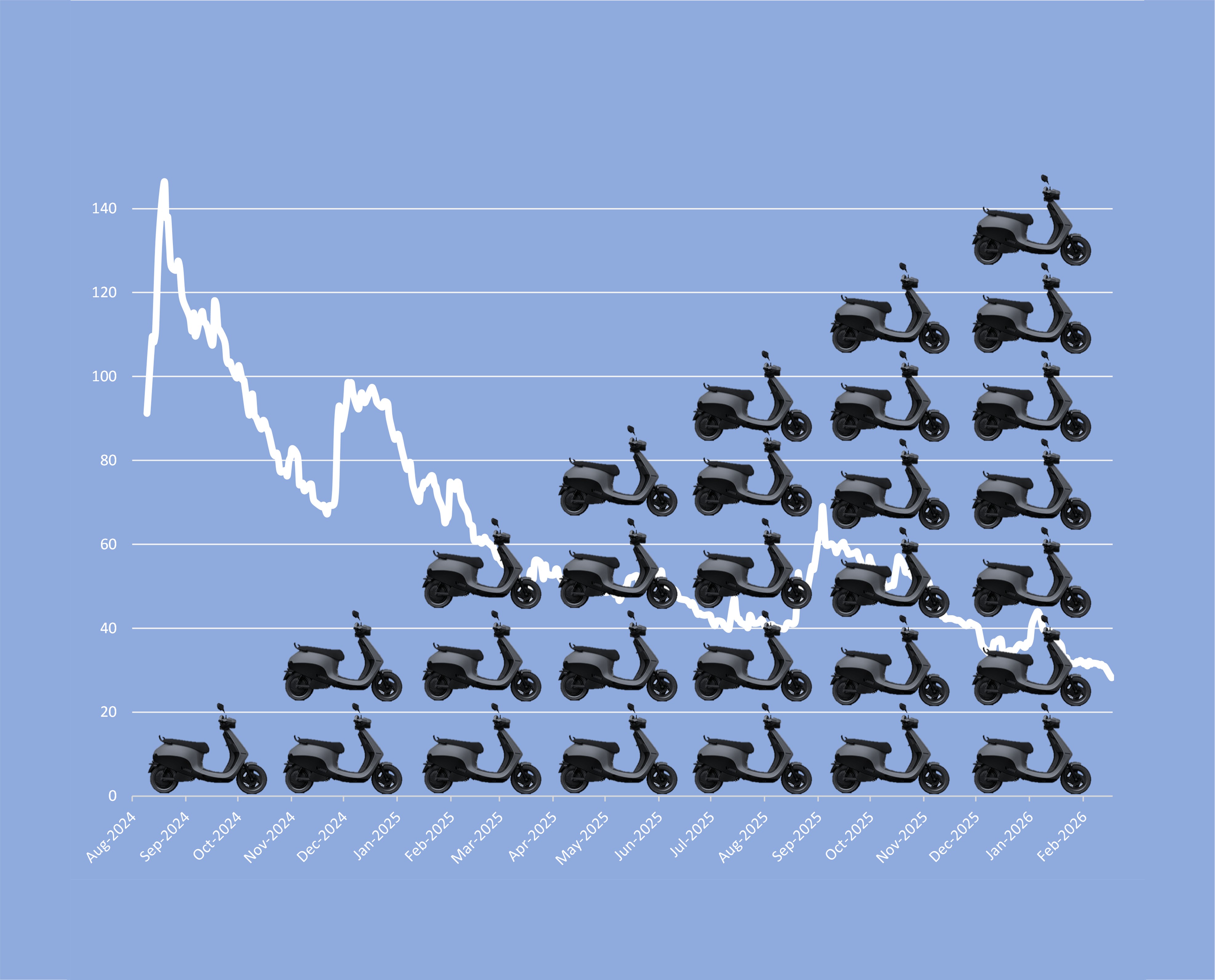
Motilal Oswal Mutual Fund’s inexplicable Ola Electric love
While its peers headed for the exit, the fund house doubled down on the falling stock. The contrarian call now looks expensive—and risky.
You may also like
Business
Mankind needs to get its mojo back
Once a stock market darling, the pharma company is bearing the brunt of a costly acquisition.
Business
Dr. Reddy’s wants to be India’s fifth largest drugmaker. What will it take?
The stock market has taken note of the company’s ambitious plans in the domestic market. But the path ahead is far from easy.
Internet
Why quick delivery of medicines will be difficult to scale
Quick-commerce apps are likely to run into regulations and poor order volumes as they try to grow this business.